DSC-TGA
DSC-TGA is an analytical tool to evaluate the behaviour of a material under different conditions (temperature and gas atmospheres). The device combines two analytical methods, thermal gravimetric analysis (TGA) and differential scanning calorimetry (DSC).
Firstly, TGA measures the mass of a sample as a function of temperature or time under different gas atmospheres. Secondly, DSC measures the amount of heat involved in a sample (endothermic or exothermic) in parallel to the TGA. Below two examples are provided to show how InsPyro uses this tool to evaluate material behavior.
Example 1 – heating and melting of sludge
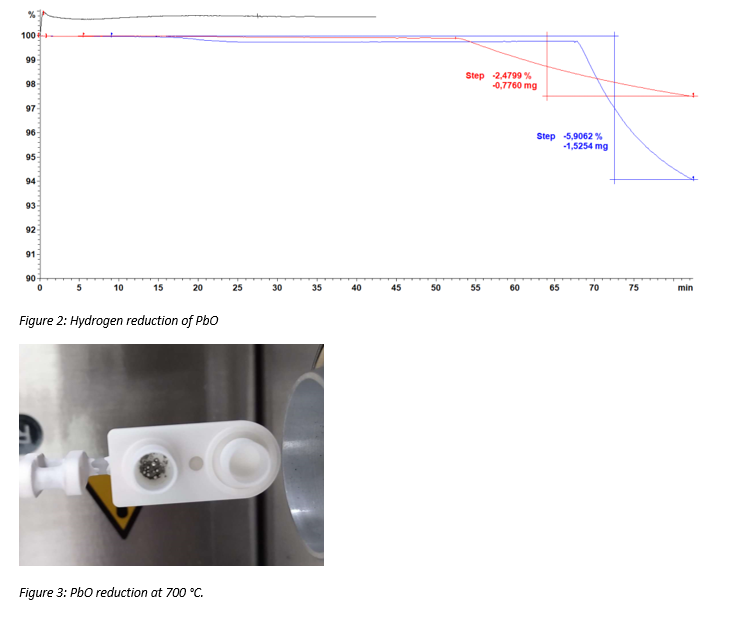
This technique allows one to evaluate the melting behavior of a material. In this particular case we evaluate a sewage sludge ash. The DSC-TGA gives the weight loss during a specific heating profile while simultaneously measuring the heat released/consumed in different reactions. Figure 1 shows the results of the analysis. On the left hand side the mass loss is observed as a function of temperature. Initially, some volatiles are released. From around 1100°C the material is melted and some minor thermal decomposition occurs such as Fe2O3 to FeO resulting in additional mass loss. The graph on the right hand side shows the energy required for the endothermic melting reaction. The melting interval of the material can also be determined using this method.
Example 2 – Reduction with H2 gas
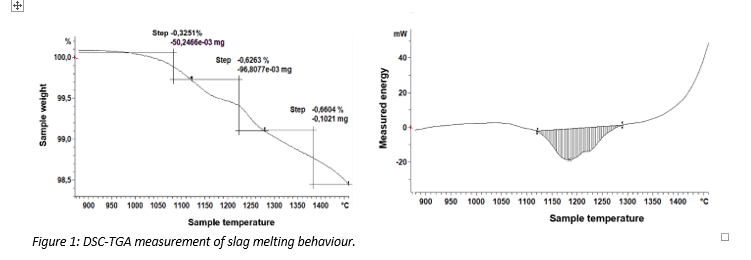
In another example the potential of hydrogen to reduce PbO is investigated. Each sample is heated up to the set temperature (300°C, 500°C, 700°C) in a N2 atmosphere (60 mL/min). The sample is then kept at the set temperature for 15 – 30 minutes in a 2% H2/Ar atmosphere (60 mL/min). Figure 2 shows the mass loss of pure PbO as a function of temperature. The following reaction occurs:
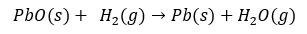
The mass loss can be used to determine the reduction that occurs. A maximum mass loss of 7% is achieved at 100% reduction. At 700 °C, 70% reduction is achieved after 15 minutes. This is confirmed by finding bright Pb metal after the trial (Figure 3).